Irma Rukhadze, PhD
Areas of Study
Sleep and respiratory neurobiology
Education
- Harvard Medical School2012Postdoctoral Training
- University of Pennsylvania2005Postdoctoral Training
- Georgian National Academy of Sciences2002PhD
Research
Dr. Rukhadze is co-leading the Sleep and Sleep Disorders Laboratory with Dr. Fenik. Dr. Rukhadze has a background in sleep and respiratory neurobiology and specific training in neuroanatomy and molecular genetics. Dr. Fenik is NIH funded investigator with a background in biophysics and physiology and expertise in in vivo electrophysiology, sleep and respiratory physiology.
The anatomical and functional interactions between the different populations of brainstem noradrenergic (NA) neurons and upper airway (UA) muscles. Brainstem NA neurons reduce their activity during Non-REM sleep and are silent during REM sleep. Withdrawal of NA excitation from UA motoneurons during sleep is the most widely supported hypothesis to explain sleep-related depression of UA motoneurons. However, which NA neurons have ability to rescue GG muscle activity from its depression during sleep, remain to be discovered. The goal of this study is to determine the functional interaction between the brainstem A1/C1, A5, LC, and SubC noradrenergic neurons and UA muscles. In these in vivo functional studies, we use a combination of chemogenetic technique permitting selective activation or inhibition of multiple brain areas and UA muscle recording during sleep and wakefulness in DBH-cre mice exposed to CIH. Alongside these studies, we also investigate the impact of CIH on anatomical projections to UA motoneurons using Channel Rhodopsin2 (ChR2)-assisted circuit mapping in transgenic mice. These studies are expected to produce a comprehensive anatomical and functional map of NA control of UA muscles that will inform future translational studies that could selectively target the NA groups as well as their afferent/efferent projections. This project is funded by NIH.
To determine the impact of aging on the functioning of brainstem NA neurons and their control of UA muscles. The prevalence of OSA is dramatically increased with age and has reached 32%-62% in older individuals as compared to 10%-15% in middle-aged population. The exact mechanisms whereby aging increases the risk of OSA are not well understood. Normal aging is associated with degeneration of NA neurons in LC, accelerated forebrain amyloid deposition and neural injury in a murine model of Alzheimer’s disease. Brainstem NA neurons that play a major role in sleep-related control of UA muscles and are critical for UA patency may undergo age-related neural injury. The objective of this study is to determine effect of aging on 1) the functional and anatomical properties of brainstem NA neurons; and 2) relationship between NA system and UA muscles measured by GG muscle activity during sleep-wake states in aging mice. In these studies, we will determine which of the group of brainstem NA neurons are most vulnerable to the age-related changes in relation to the control of UA muscles and the effect of aging on the density of NA innervation of hypoglossal motoneurons that innervate the upper airway muscles. This project is funded by NIH.
Determine the sleep-related changes in genioglossus muscle activity in behaving DBH-cre mice.
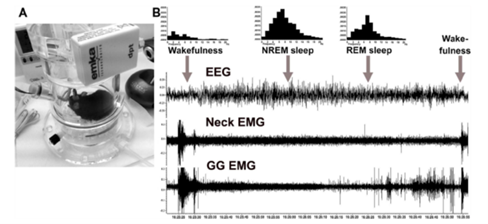
Investigate the contribution of A/C1 noradrenergic/adrenergic neurons to sleep-related depression of genioglossus muscle activity using the Inhibitory DREADD (hSyn-DIO-hM4Di-mCherry).
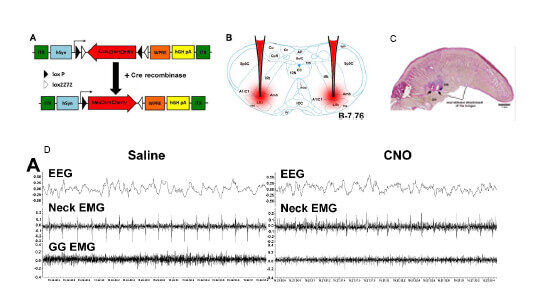
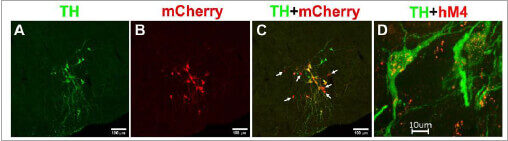
Employ immunohistochemistry to complement the chemogenetic approach and determine expression of DREADD in noradrenergic, TH-positive neurons.
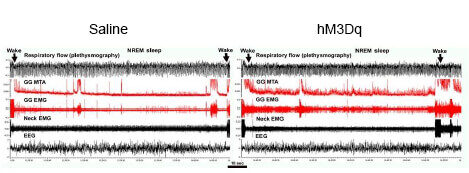
Investigate the contribution of A7 noradrenergic neurons to sleep-related depression of genioglossus muscle activity using the excitatory DREADD (hSyn-DIO-hM3Dq-mCherry).
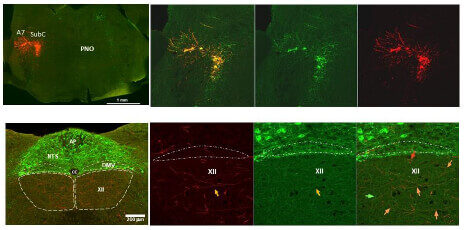
Determine sources of noradrenergic and cholinergic excitatory drive to hypoglossal motoneurons (XII) that innervate the upper airway muscles.
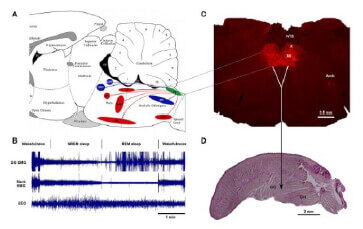
Determine the effect of chronic intermittent hypoxia (CIH), a major pathogenic factor of OSA, on noradrenergic innervation of XII motor motoneurons using the Channel Rhodopsin2 (ChR2)-assisted tracer in DBH-cre mice exposed to CIH, an animal model of OSA.
OSA is a chronic disorder caused by repeated upper-airway collapse during sleep, resulting in episodes of apnea and arousals that result in fragmented sleep and intermittent hypoxia/reoxygenation, hypercapnia, and increased sympathetic activation. In OSA patients, the hypoxic burden in addition to sleep deprivation is associated with further molecular and cellular disruptions, leading to oxidative stress, neuronal injury and apoptosis. These key injurious stimuli give rise to major cardiovascular, metabolic, cognitive and neurodegenerative disorders, and the progression of cancer. OSA is more prevalent in men than in women and its prevalence increases with age. The major contributor to OSA pathophysiology is the sleep-related reduction of the tone in upper airway muscles, mainly the genioglossus (GG) muscle which is the major upper airway dilator.
We focus to define neural connections to upper airway motoneurons and neurochemical mechanisms that are responsible for upper airway collapse during sleep in young and elderly population, and identify neuronal targets for therapeutic approaches to develop pharmacological treatment and novel interventional strategies for OSA. To this end we use chronic intermittent hypoxia (CIH) a mouse model of OSA, recording of sleep and wakefulness in behaving mice, recording of upper airway muscle activity during sleep and wakefulness, plethysmography, neuronal tracing (AAV-EF1a-DIO-hChR2(H134R)-mCherry and retrograde tracers), chemogenetics (inhibitory-hSyn-DIO-hM4Di-mCherry & excitatory-hSyn-DIO-hM3Dq-mCherry DREADDs), Cre Driver lines of transgenic mice, electrophysiology and neuropharmacology.
The long-term goal of our laboratory is to determine the underlying mechanisms of:
- Pathophysiology of obstructive sleep apnea (OSA)
- Neural mechanisms of age-related increase in OSA prevalence
- Sex differences in OSA
- How OSA contribute to age-related cognitive deficits and neurodegeneration
- To develop pharmacological therapies and gender-specific interventional strategies for OSA treatment and prevention of its comorbidities.
Publications
View Irma Rukhadze's articles on the National Institute of Health's PubMed website.

