Areas of Study
Cardiovascular and respiratory muscle biology
Education
- Fourth Military Medical University, China1990
- Wannan Medical College, China1983
Research
Our interests focus on basic, translational, and drug discovery research in cardiovascular and respiratory muscle biology and diseases.
We have extensive research experience in the studies of the molecular geneses, regulatory mechanisms, signaling pathways, and critical functions of cell calcium, ion channels, reactive oxygen species, neurotransmitter receptors, protein kinases, and non-coding RNAs using molecular, biochemical, physiological, pharmacological, and genetic approaches at the molecular, organelle, cellular, tissue and organism levels in animals and human samples.
As a Principal Investigator, I have received multiple research grants from the National Institutes of Health, a Scientist Development Grant and Established Investigator Award from the American Heart Association, a Research Award from the American Diabetes Association, and others.
As corresponding author, first author, and key contributor, I have had numerous publications in highly peer-reviewed journals including Nat Commun (impact factor: 12.121), Antioxid Redox Signal (8.209), Proc Natl Acad Sci USA (9.432), Free Radic Biol Med (6.081), Cell Calcium (4.288), J Gen Physiol (4.260), J Biol Chem (5.328), FASEB J (6.515), Nature (34.480), Circ Res (9.214), etc. I have been the editor of academic books, most recently Lung Inflammation in Health and Disease, Volume I and II, published as part of the Advances in Experimental Medicine and Biology book series by Springer (New York) in April and May 2021, respectively.
I have also served as an editorial board member and/or section editor for the Clinical and Translational Medicine, Pulmonary Circulation, and several other journals, as well as the executive committee member and/or subcommittee chair for the International Society of Translational Medicine and other professional societies.
Calcium and reactive oxygen species signaling in hypoxic pulmonary vascular physiology and hypertension
Intracellular Ca2+ release from the sarcoplasmic reticulum (SR) in smooth muscle cells (SMCs) plays a crucial role in hypoxia-induced pulmonary vasoconstriction, vasoremodeling, and hypertension. However, the cellular and molecular mechanisms coupling hypoxia to Ca2+ release remain incompletely understood. In this research project, we attempt to determine the primary oxygen sensor, key signaling elements, and essential effector molecules that mediate the role of Ca2+ release in hypoxic pulmonary vasoconstriction, vasoremodeling, and pulmonary hypertension. Specifically, we have been focusing on mitochondrial molecules, NADPH oxidase, reactive oxygen species (ROS), FK506 binding protein 12.6 (FKBP12.6), cyclic ADP-ribose, nicotinic acid adenine dinucleotide phosphate, protein kinases, ryanodine receptor (RyR), inositol triphosphate receptor (IP3R), inflammatory transcriptional factors, and non-encoding RNAs in pulmonary artery smooth muscle cells (PASMCs).
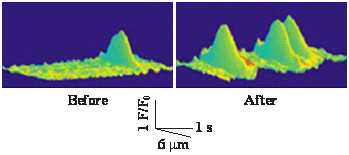
Effect of mild hypoxia on ryanodine receptor (RyR) Ca2+ release channel activity, determined by measuring RyR-generated Ca2+ spark, in freshly isolated mouse pulmonary arterial smooth muscle cells (PASMCs) using a laser scanning confocal microscope. The result reveals that hypoxia significantly increases Ca2+ spark frequency (i.e., RyR channel activity) in PASMCs.
Novel neurotransmitter and transcriptional signaling in respiratory muscle biology and diseases
Cholinergic nerves predominately control airway smooth muscle cells (ASMCs) through muscarinic receptors. Stimulation of these receptors by their agonists activates phospholipase C and generates inositol triphosphate (IP3), which induces Ca2+ release through IP3 receptor (IP3R). This research project seeks to examine the interactive roles of muscarinic neurotransmitter receptors with IP3R and ryanodine receptor (RyR) Ca2+ release channels, transient receptor potential canonical (TRPC) cation channels, inflammatory factors, transcription factors, and non-coding RNAs in respiratory muscle biology and disease states (e.g., asthma and chronic obstructive pulmonary disease).
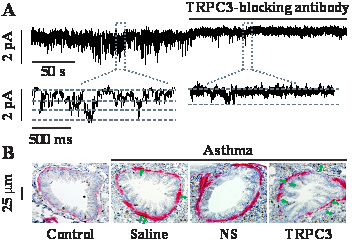
A: Transient receptor potential canonical 3 (TRPC3) channel was highly expressed in airway smooth muscle cells (ASMCs). Constitutively active cation channel activity was measured in a mouse ASMC membrane patch before and after application of specific TRPC3 channel-blocking antibodies using the inside-out single channel recording. TRPC3 channel-blocking antibodies fully blocked the channel activity, demonstrating TRPC3 channel in ASMCs.
B: Immunohistochemistry staining α-smooth muscle actin (pink) and Ki67 (brown) in airways from control and asthmatic mice untreated and treated with saline, lentiviral SM22α promoter-driven non-silencing (NS) shRNA or TRPC3 channel shRNA in vivo. Green arrows indicate colocalization of a-smooth muscle (ASM) actin and Ki67. The result unveils that ASM layer and Ki67-positive cells were both completely blocked, indicating the important role of TRPC3 channels in airway muscle remodeling and associated asthma states.
Calcium, ROS and their upstream/downstream signaling in diabetic vascular and neural complications
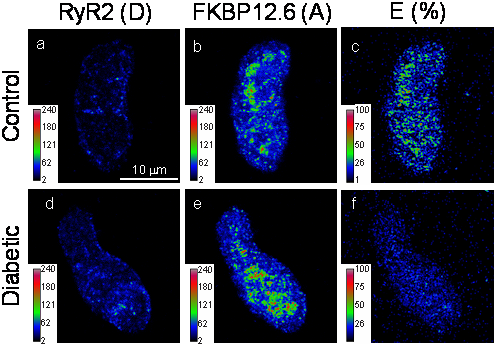
Diabetes is a common disease with devastating cardiovascular and neural complications. Current medications for vascular and neural complications are nonspecific and may have limited efficacy. A main reason for this gap is that the underlying mechanistic molecular processes are poorly understood. Herein, we seek to address this unmet need by determining the potential important roles of FK506 binding protein 12.6 (FKBP12.6), big-conductance K+ (BK) channel, ryanodine receptor (RyR) Ca2+ release channel, mitochondrial reactive oxygen species (ROS), inflammatory transcription factors, as well as their upstream and downstream signaling molecules in the development of hypertension and vascular dementia in diabetes.
Immunofluorescence images from a control and diabetic mouse CASMC (stained with anti-RyR2 (panels a-c; D, donor) and anti-FKBP12.6 bodies (panels d-f; A, recipient) were collected using Zeiss LSM510 microscopy and processed using the PFRET algorithm to remove spectral bleed-through. Pseudocolor images depict the pixel-by-pixel distribution of D (a & d), A (b & e) and FRET (E(%), c & f) levels.