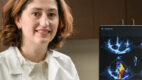
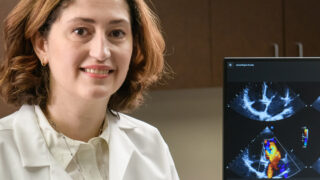
Very Best Heart Care from Our Region's Academic Health System
The sensation of nagging heartburn was keeping Irene Warenchak awake at night. It seemed nothing she did would make it go away. Eventually, a close friend encouraged her to see a doctor. So she made an appointment with a gastroenterologist. It turned out that her persistent pain was not caused by...